Looking for a stool donor for your do-it-yourself FMT (fecal microbiota transplant)? Breastfed baby poop may be some of the best untapped medicine available. Use of children’s feces as donor material is part of FMT history with beginnings in fourth century China.
The Gut Club can help you evaluate FMT donor material through microbial DNA stool testing for optimal matching of donor with receiver. This is part of our Microbiome Balancing Private Consult.
Currently, the FDA allows doctors to perform fecal microbiota transplant (FMT) for C. difficile patients only. Yet research reveals FMT can apply to many different health problems, both mental and physical. Emerging FMT applications include recovering children from autism, inflammatory bowel disease (IBD includes ulcerative colitis and Crohn’s disease), irritable bowel syndrome (IBS), autoimmune and allergic disease, metabolic syndrome, Parkinson’s disease, multiple sclerosis (MS), chronic fatigue syndrome (CFS), Alzheimer’s disease, epilepsy, longevity and obesity. Perhaps FMT can also apply to those suffering from a vaccine injury. Fecal microbiota transplantation broadening its application beyond intestinal disorders (2015)
Why Breasted Baby Poop? You may want to strongly consider using unvaccinated BREASTFED BABY POOP for the following reasons:
- Healthy phages. Phages may be key to FMT success rate. Fresh stool may be best as freezing can deactivate and injure phages.
- Pre-weaned is high Bifidobacteria which may be a positive predictor for successful FMT.
- Post-weaned is high butyrate-producing Clostridia which may contribute to successful FMT.
- Some level of safety due to extensive routine testing for maternal health in pregnancy which may indicate infant health.
- Protective, immune-regulating Bacteroides.

Why unvaccinated baby poop?
In theory, unvaccinated baby poop may be best to avoid potential for detrimental flora shift in donor material. There are currently no published research papers detailing flora shift caused by vaccination, with one exception where the cholera vaccine was found to significantly raise gram-negative bacteria. It is conceivable that a microbial predisposition to flora shift by vaccination takes place via viral-bacterial interaction with receptors shared by both viruses and bacteria, i.e., SLAM and CD46 in measles. These receptors are also responsible for host immune response to gram-negative bacteria where this dynamic may lead to immunosuppression, i.e., neutrophil activation or neutropenia. This is part of our Microbiome Vaccine Safety Project. New paper: Do gut microbiota mediate adverse vaccine reaction?
To have your prospective donor material evaluated, please see the following page:
Microbiome Balancing Private Consult

Here is our ongoing exploration about factors related to successful FMT:
UPDATE 8.21.19:
March 2019 Case Report:
The Effects of Fecal Donors with Different Feeding Patterns on Diarrhea in a Patient Undergoing Hematopoietic Stem Cell Transplantation https://www.hindawi.com/journals/crihem/2019/4505238/
“Here, we found that 1-year-old infants can serve as safe FMT donors for treating diarrhea in HSCT patients. Furthermore, the overall diversity of the intestinal microbiota may not be the only important element in the selection of an effective FMT donor. The richness of probiotics, including Lactobacillus, Bifidobacteria, and Saccharomyces boulardii, might be the key factor in choosing the right FMT donor”
“As we know, adults have a more complex diet than that of 1-year-old infants. Thus, for safety reasons, we used feces from infants instead of adults to perform FMT. Infants 1 year of age have more Bifidobacteria than do individuals of other age groups [34]. Several trials have suggested that Bifidobacterial preparations have efficacy in the prevention and treatment of pediatric antibiotic-associated diarrhea [35]. Importantly, the infant intestinal microbiome is significantly associated with the feeding method [36]. This raises the question of what types of infant donors are better suited for FMT. Our study attempts to solve this problem by using feces from two infants with different dietary patterns.”
“Fecal samples were obtained from a 1-year-old female infant (donor #1) who had a mixed feeding pattern (formula feeding and complementary food). Doses of 60 mL of fecal suspension were administered for 7 consecutive days via a nasoduodenal tube. en, we suspended the FMT operation and continually observed the dynamic changes in the intestinal microbiota. However, one week after cessation of FMT, diarrhea recurred in this patient. Unfortunately, stool samples from donor #1 were not available on that occasion. us, we used fecal samples from another 1-year- old male infant (donor #2) who had an exclusive breastfeeding pattern. After 3 consecutive days of FMT2nd, we did not observe any improvement in the diarrhea, and we terminated FMT on day 4 (Figure 1(a)). Two days later, we obtained additional stool samples from donor #1 and conducted the third FMT (FMT3rd) for another 7 consec- utive days.”
“we also found that the bacteria of the two infant donors were largely restricted to a small subset of the bacterial world, namely, Bifidobacteria, Streptococci, and Lactobacillus spp. Increasing evidence has now demonstrated that some microbial species (e.g., Lactobacilli and Streptococcus) can restore the disrupted gut epithelium that causes the diarrhea commonly experienced by HSCT patients through upregulation or phosphorylation of tight junction proteins [42–44]. Despite this, there was an obvious difference in diarrhea treatment outcomes between these two FMT donors. Through a small FMT substitution, we observed that stool volume and frequency were reduced when we used feces from donor #1, but these effects were not observed after the transplant from donor #2. Our and previous studies have shown that not all FMT is fully effective. Thus, choosing an appropriate FMT donor is particularly important for clinicians when they use FMT to treat diarrhea.”
“For the donors, we observed that Bifidobacterium (29.8%) and Faecalibacterium (28.8%) were the dominant genera in donors #1 and #2, respectively. Other highly represented genera in both donors included Lactobacillus (donor #1 = 16.3%, donor #2 = 2.9%), Veillonella (donor #1 = 15%, donor #2 = 4.6%), Streptococcus (donor #1 = 6.6%, donor #2 = 4.8%), Haemophilus (donor #1 = 4.6%, donor #2 = 6.2%), and Escherichia-Shigella (donor #1 = 11.3%, donor #2 = 0.6%).”
UPDATE 8/22/20:
Diversity, compositional and functional differences between gut microbiota of children and adults
https://www.nature.com/articles/s41598-020-57734-z
“The comparison of the two cohorts showed that children had significantly lower gut microbiome diversity. Furthermore, we observed higher relative abundances of genus Bacteroides in children and higher relative abundances of genus Blautia in adults. Predicted functional metagenome analysis showed an overrepresentation of the glycan degradation pathways, riboflavin (vitamin B2), pyridoxine (vitamin B6) and folate (vitamin B9) biosynthesis pathways in children. In contrast, the gut microbiome of adults showed higher abundances of carbohydrate metabolism pathways, beta-lactam resistance, thiamine (vitamin B1) and pantothenic (vitamin B5) biosynthesis pathways. A predominance of catabolic pathways in children (valine, leucine and isoleucine degradation) as compared to biosynthetic pathways in adults (valine, leucine and isoleucine biosynthesis) suggests a functional microbiome switch to the latter in adult individuals.”
UPDATE 8/3/22:
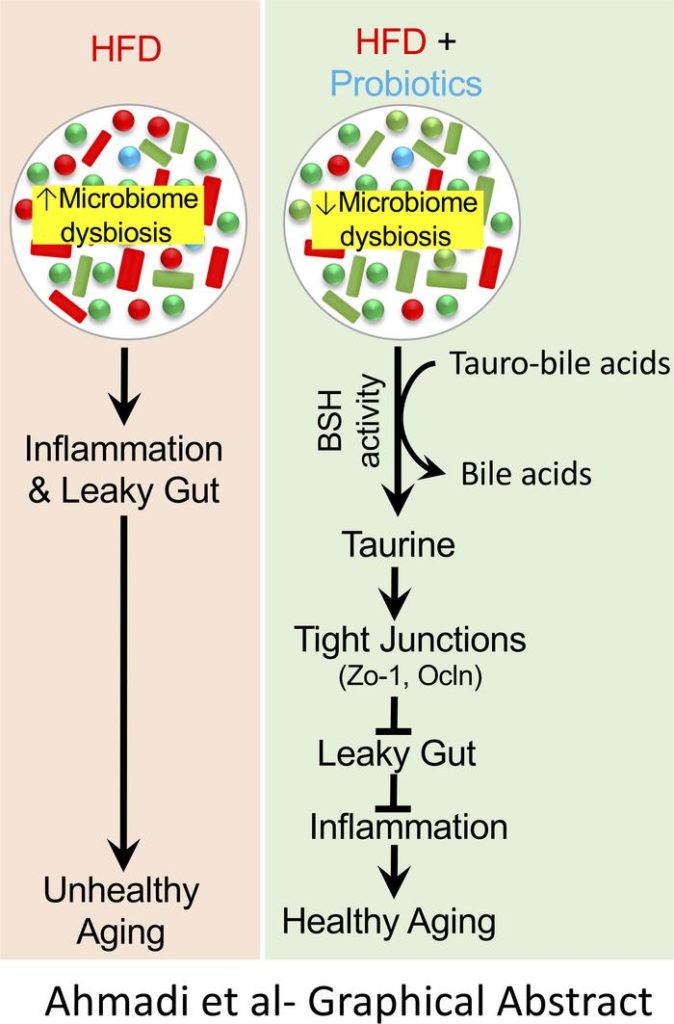
A human-origin probiotic cocktail ameliorates aging-related leaky gut and inflammation via modulating the microbiota/taurine/tight junction axis
“human-origin probiotic cocktail containing 5 Lactobacillus and 5 Enterococcus strains isolated from healthy infant gut prevented high-fat diet–induced (HFD-induced) microbiota dysbiosis, leaky gut, inflammation, metabolic dysfunctions, and physical function decline in older mice.” (2020)
https://insight.jci.org/articles/view/132055
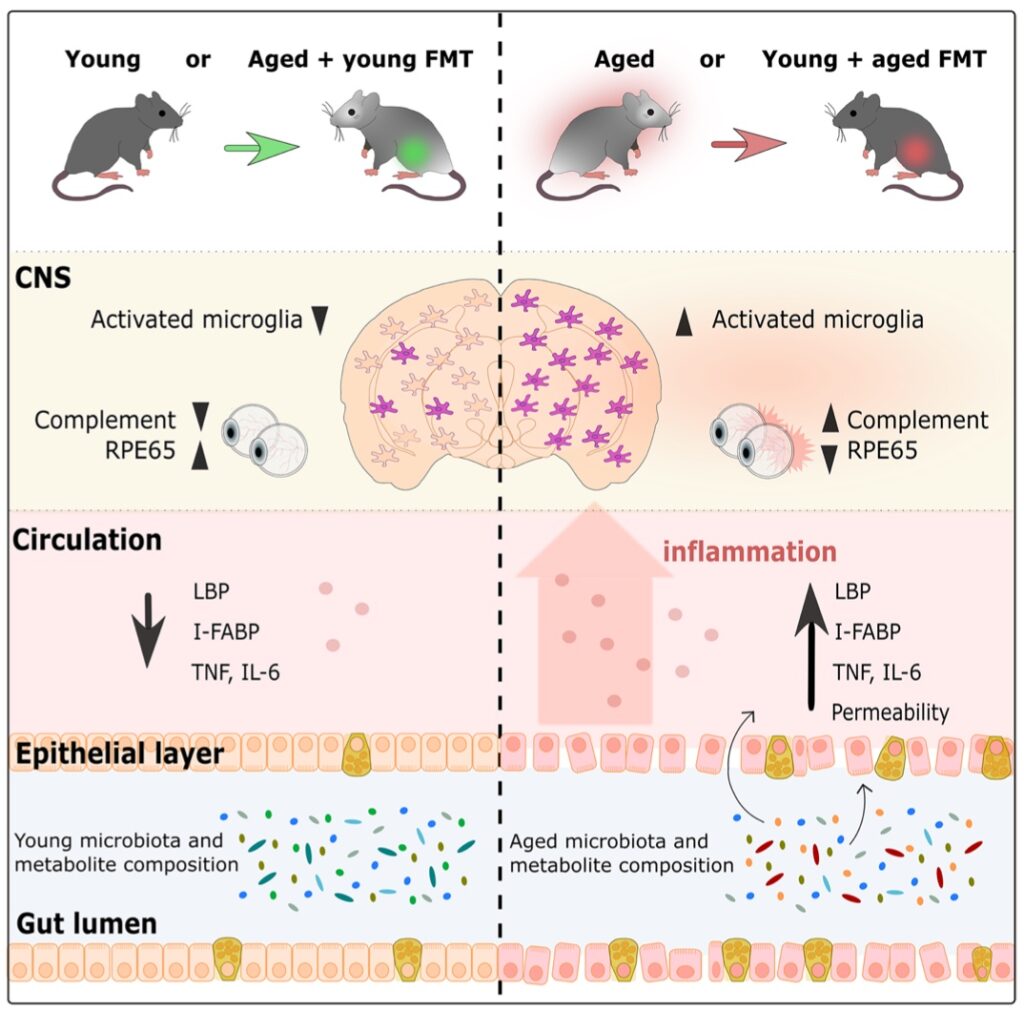
YOUNG FMT DONOR DOES IT AGAIN:
‘Fecal microbiota transfer between young and aged mice reverses hallmarks of the aging gut, eye, and brain’ (2022)
https://microbiomejournal.biomedcentral.com/articles/10.1186/s40168-022-01243-w
Update 8.12.22
April 2022:
‘Fecal microbiota transfer between young and aged mice reverses hallmarks of the aging gut, eye, and brain’
https://microbiomejournal.biomedcentral.com/articles/10.1186/s40168-022-01243-w
July 2022:
‘Young fecal transplantation mitigates the toxicity of perfluorobutanesulfonate and potently refreshes the reproductive endocrine system in aged recipients’
https://www.sciencedirect.com/science/article/pii/S0160412022003452
June 2022:
‘Disturbed glucose metabolism by perfluorobutanesulfonate pollutant and benefit of young fecal transplantation in aged zebrafish’
https://www.sciencedirect.com/science/article/pii/S0147651322005619
July 2022:
‘Rejuvenating the human gut microbiome’
https://www.sciencedirect.com/science/article/pii/S1471491422001137
May 2024:
Fecal microbiota transplantation from young mice rejuvenates aged hematopoietic stem cells by suppressing inflammation
https://ashpublications.org/blood/article/141/14/1691/494137/Fecal-microbiota-transplantation-from-young-mice
June 2024:
‘Probiotic lactobacilli in faeces of breastfed babies’
“27 strains were identified representing 104 species of Lactobacillus isolated from baby feces.”
June 2024:
‘Probiotic lactobacilli in faeces of breastfed babies'”27 strains were identified representing 104 species of Lactobacillus isolated from baby feces.”
“Almost all strains showed antibacterial activity against the enteric pathogens Escherichia coli O157:H7, E. coli, Listeria monocytogenes, Staphylococcus aureus, and Salmonella Enteritidis. In this research, lactobacilli isolated from babies had probiotic properties.”
https://www.scielo.br/j/cta/a/5gTV3VQNCmvtKZNqtd8yTqH/?lang=en